Abubuwan Alkarine ba su da caji ba mai daukar hoto ba, jigon batir ba shi da caji.Jakadan AT AC ADD ACD, kuma ana kiranta da batirin VRLA, sun bambanta cikin girma kuma galibi guniid ne, kuma galibi ana amfani da su don fara ajiyar garken wuta don manyan motoci. Alkaline batura gaba daya karami ne da silili a cikin girman.
Jagorar acid acidatul wani irin baturi ne wanda ke da ƙarfin lantarki fiye da baturin alkaline. Babban ƙarfin lantarki yana ba da damar ƙarfin motocin lantarki tare da ƙarin iko, kuma yana ba ka damar amfani da ƙarancin kuɗaɗe yayin aiki na'urorin lantarki.
Menene jagorancin acid acid?
Kwayoyin a cikin jagorar acid na acid za a iya ambaliya ko a cikin hanyar gel, kuma wani babban bambanci ne tsakanin baturin acid da batirin alkalami shine babban baturin acid yana da ƙarfin lantarki. Babban ƙarfin lantarki yana ba shi damar zuwa motocin lantarki tare da ƙarin iko. Hakanan ana san batir na acid acid da sel na rigar kuma suna shigowa ko ɗayan nau'ikan tantanin geel.
Baturin acid acid wani nau'in nebatir mai cajiWannan yana amfani da faranti na jagorori da lantarki azaman tushen makamashi. Baturin acid acidat baturin yana da babban ƙarfin kuzari fiye da sauran nau'ikan batir, wanda ya sa ya zama mafi iko da inganci. Baturin acid acid batir wani nau'in batir ne wanda yake amfani da faranti a matsayin kayan aikinsu. Ana amfani dashi a cikin motoci, jiragen ruwa da sauran motocin.
Baturin acid acid batirin wani nau'in batir ajiya. Baturin acid na acid batir sun shahara sosai saboda suna da inganci, ingantacce, kuma mai sauƙin amfani.
Menene batirin alkaline?
Bilo na alkaline wani ɗan batir ne wanda yake amfani da zinc chloride a matsayin wayewarta maimakon maganin alkaline. Wannan yana sa Alkaline Bater mafi aminci da ƙari masu zaman kansu suna da alaƙa da baturin na al'ada a acid.
Baturi na alkaline ne wanda ya ƙunshi kayan aiki mai aiki wanda ya ƙunshi gishirin alkali, potassium hydroxide) da oxide. Hakanan za'a iya kiran batura mara izini ko bushewa saboda ba sa buƙatar kowane kulawa bayan amfani.kaline Batura a na'urori daban-daban, haɗi da kyamarori. Sun kasance suna zagaye shekaru da yawa kuma za su kasance a kusa da mutane da yawa.
Bambance-bambance a cikin kayan batir:
1.Baturin acid na acid ya ƙunshi faranti na ƙasa, waɗanda aka yi da biji da sulfuric acid. Wadannan fararen faranti suna rufaffen a cikin wani akwati da ake kira sel. Lokacin da ka cajin baturin, sulfuric acid ya yi aiki tare da faranti na jirgin sama don samar da wutar lantarki. Wannan tsari an san shi da lantarki.
2.Abubuwan da alkaline sun ƙunshi zinc da kuma moganese dioxide a cikin wayewar su. Wadannan kayan suna amsawa tare da abubuwan lantarki (tabbatacce da mara kyau) don samar da wutar lantarki yayin caji ta amfani da caja.
3.Baturin ya ƙunshi wayoyin lantarki guda biyu da electrolyte. Ana kiran ingantacciyar lantarki mai kyau, kuma ana kiransa mummunan outsionin mara kyau. A cikin baturi, ions motsi daga ɗaya elecrode zuwa wani lokacin da kuka yi amfani da karamin adadin wutar lantarki. Ana kiran wannan motsi mai ƙarfi (EMF).
4.Baturin ya ƙunshi wayoyin lantarki guda biyu da electrolyte. Ana kiran ingantacciyar lantarki mai kyau, kuma ana kiransa mummunan outsionin mara kyau. A cikin baturi, ions motsi daga ɗaya elecrode zuwa wani lokacin da kuka yi amfani da karamin adadin wutar lantarki. Ana kiran wannan motsi mai ƙarfi (EMF).
5.Sakamakon batirin ya samar da sakamakon baturin daga wannan EMF wanda ke haifar da motsi tsakanin wayoyin da ta yi.
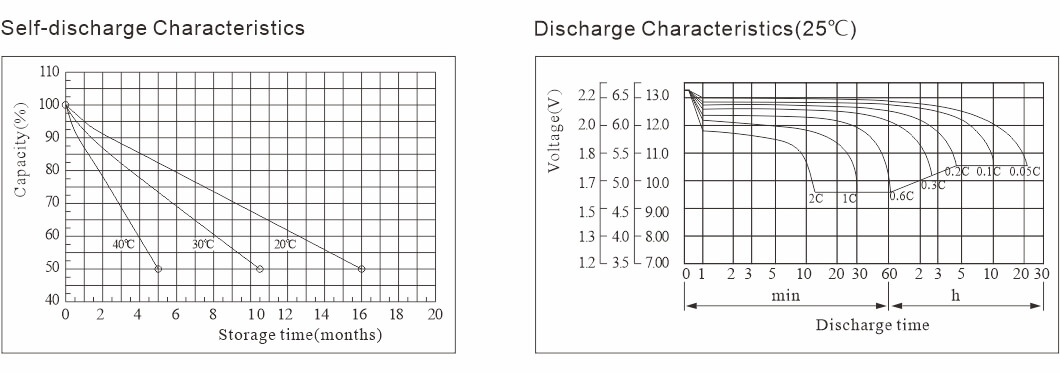
Bambancin batirin:
Abubuwan da alkaline sun dace da ci gaba da fitarwa da kuma babban aikin lantarki, ya dace da kyamarori, kayan rubutu, masu nisa, mabuɗin mabuɗin, ƙamus, shayarwa, da sauransu.
Batirin Of At-acid sun dace da filayen ikon, kamar baturan wutar lantarki, kayan haɗin lantarki, jerin batir, UPSTO Batoran batir.
Ba a ce wane baturi ya fi kyau ba. Kowane nau'in baturi yana da kewayon aikace-aikacen. Zai fi kyau cikakke don zaɓar batir da ya dace don filayen daban-daban.
Rayuwar AlKaline:
Akwai baturan alkaline a cikin iri-iri da kuma voltages. Suna da rayuwar shiryayye har zuwa shekaru 10, idan aka kwatanta da shekaru 3 don daidaitattun batir.
Jagorar Attaukar Acidan Baturin Att Acid:
Rayuwar ƙira na ƙira na batirin-acid shine shekaru 3-5 kuma fiye da shekaru 12, amma wannan rayuwar sabis ne. Akwai bambance-bambance tsakanin ainihin rayuwar sabis da ka'idar. Kuna buƙatar kula da baturinku na acid dinka gwargwadon iko don tabbatar da cewa yana da mafi ƙarancin rashi.
Yanayin Aikaceos:
Baturin acid na acid sune mafi yawan nau'ikan batir da aka yi amfani da shi a cikin motoci da sauran aikace-aikace. Za'a iya siyan waɗannan baturan daga kusan duk wani dillali ko kan layi, gwargwadon girman da nau'in da kake so.
Cikakken tsarin kula da baturi na acid na iya komawa zuwa labarin:
Jagoran mai amfani da kayan baturi na acid
Babban bambanci tsakanin waɗannan nau'ikan batir guda biyu shine adadin kuzarin da aka adana a kowane yanki mai nauyi. Baturin acid Acidat baturin yana da babban ƙarfin lantarki, wanda yake nufin ƙarin iko don motarka don motsa shi da sauri ko amfani azaman tsarin madadin lantarki don gidanka / kasuwanci. Jagoran batirin acid suma suna da tsayi fiye da abin da alkaline, amma saboda ba sa samar da makamashi da yawa a kowane yanki na nauyi, sun fi tsada sosai!
Lokaci: Jul-11-2022




