Amabhethri e-Alkaline ikakhulukazi angabizi, amabhethri e-lead-acid ayaphindeka.Amabhethri e-lead-acid, eyaziwa nangokuthi amabhethri e-VRLA, ayahlukahluka ngosayizi futhi iningi lawo i-Culoid, futhi asetshenziselwa kakhulu ukuqala okulondolozwa kwamandla ezimoto ezinkulu. Amabhethri e-Alkaline ngokuvamile ancanyana futhi asayizi ngosayizi.
Ibhethri le-lead acid luhlobo lwebhethri olunamandla kagesi aphezulu kunebhethri le-alkaline. I-voltage ephakeme ivumela ukuthi inikeze izimoto zikagesi ezinamandla ngamandla amaningi, futhi futhi ikuvumela ukuthi usebenzise amandla amancane lapho usebenza amadivaysi kagesi.
Yini ibhethri le-acid eliholayo?
Amaseli ebhethri le-lead acid lingakhukhulwa noma ngefomu le-gel, futhi kwesinye isikhathi abizwa ngokuthi amabhethri "weseli" we-main I-voltage ephakeme ivumela ukuthi inikeze izimoto zikagesi ezinamandla ngamandla amaningi. Amabhethri e-lead acid abizwa nangokuthi amaseli amanzi futhi afike ngezinye izinhlobo zeseli noma ze-gel cell.
Ibhethri le-lead acid luhlobo lweIbhethri elishajekayoLokho kusebenzisa amapuleti asuselwa ku-lead kanye ne-electrolyte njengomthombo wamandla. Ibhethri le-acid eliholayo linobuningi obukhulu bamandla kunezinye izinhlobo zamabhethri, okwenza kube namandla ngokwengeziwe futhi kusebenze kahle. Ibhethri le-lead acid luhlobo lwebhethri elivuselelwa kabusha elisebenzisa amapuleti okuhola njengezinto zabo ezisebenzayo. Isetshenziswa kakhulu ezimotweni, ezikebheni nakwezinye izimoto.
I-Holy acid Battery uhlobo lwebhethri lesitoreji. Ukuhola amabhethri e-acid kudumile kakhulu ngoba abiza kakhulu, athembekile, futhi kulula ukuyisebenzisa.
Yini ibhethri le-alkaline?
I-Alkaline Battery uhlobo lwebhethri elivuselelwa kabusha elisebenzisa i-zinc chloride njenge-electrory yayo esikhundleni sekhambi le-alkaline. Lokhu kwenza ibhethri le-alkaline liphephe futhi libe nobungane ngokwemvelo kunebhethri lendabuko le-acid.
I-Alkaline Battery iyiseli le-electrochemical eliqukethe i-electrolyte esebenzayo equkethe usawoti wensimbi we-alkali (i-potassium aydroxide) ne-oxide (potassium oxide). Kungabizwa nangokuthi amabhethri e-sel-engekho emthethweni noma owomile ngoba awadingi ukulungiswa ngemuva kokusetshenziswa kwamabhethri we-serke.alkaline asetshenziswa kumadivayisi amaningi ahlukene, kufaka phakathi amakhamera namakhamera. Bebelokhu bedlula iminyaka eminingi futhi bazobe bekhona kwabaningi abaningi.
Umehluko ekubukeni kwebhethri:
1.Amabhethri e-Holid acid aqukethe amapuleti aholayo, enziwe ngokuhola kanye ne-sulfuric acid. Lawa mapuleti afakwe esitsheni esibizwa ngokuthi iseli. Lapho ukhokha ibhethri, i-sulfuric acid iphendula ngamapuleti aholayo ukukhiqiza ugesi. Le nqubo yaziwa njenge-electrolysis.
2.Amabhethri e-Alkaline aqukethe i-zinc ne-manganese dioxide ku-electrolyte yawo. Lezi zinto zokwakha ziphendule nge-electrodes (izigxobo ezingezinhle nezingalungile) ukukhiqiza ugesi lapho kufaneleka kusetshenziswa ishaja.
3.Ibhethri liqukethe ama-electrodes amabili kanye ne-electrolyte. I-electrode enhle ibizwa nge-anode, futhi i-electrode engemihle ibizwa ngokuthi i-cathode. Ebhethri, ama-Ion asuka ku-electrode uye kwelinye lapho usebenzisa inani elincane likagesi. Le nhlangano ibizwa nge-electromotive Force (EMF).
4.Ibhethri liqukethe ama-electrodes amabili kanye ne-electrolyte. I-electrode enhle ibizwa nge-anode, futhi i-electrode engemihle ibizwa ngokuthi i-cathode. Ebhethri, ama-Ion asuka ku-electrode uye kwelinye lapho usebenzisa inani elincane likagesi. Le nhlangano ibizwa nge-electromotive Force (EMF).
5.Amandla kagesi akhiqizwa yibhethri imiphumela evela kule i-EMF ebangela ukunyakaza phakathi kwama-electrodes awo.
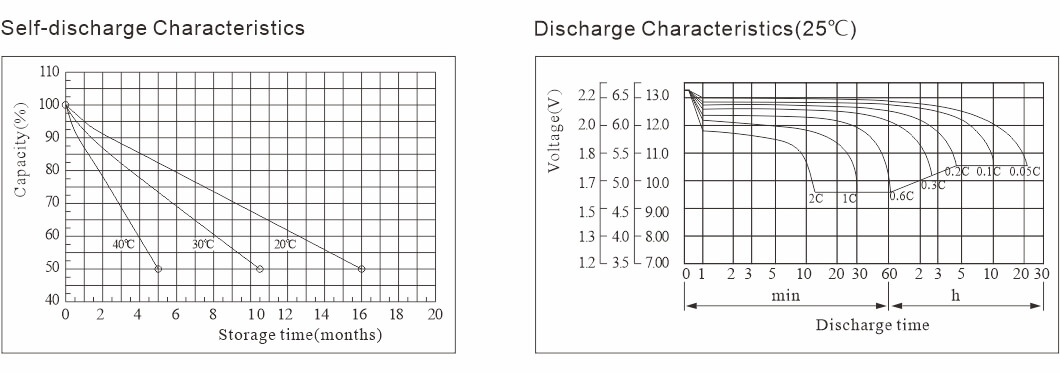
Umehluko wesicelo sebhethri:
Amabhethri e-Alkaline alungele ukukhishwa okuqhubekayo kanye nomsebenzi ophakeme we-voltage, elungele amakhamera, amathoyizi kagesi, izilawuli ezikude, amakhibhodi, amakhibhodi, ama-shaver, njll.
Amabhethri e-lead-acid alungele ama-Power Fields, njengamabhethri okusebenza kwezithuthuthu, amabhethri kagesi ezimoto, amathoyizi kagesi emkhakheni wokugcina amandla, ama-Electric Golf Cart, uchungechunge lwebhethri lwamandla, njll.
Akushiwo ukuthi yiliphi ibhethri elingcono. Uhlobo ngalunye lwebhethri lunebanga layo lokuxhumana. Kuhle kakhulu ukukhetha ibhethri elifanelekile lezinkambu ezahlukene.
Impilo yebhethri ye-Alkaline:
Amabhethri e-Alkaline ayatholakala ngosayizi ahlukahlukene kanye nama-voltages ahlukahlukene. Banempilo eshalofini efinyelela eminyakeni eyi-10, uma kuqhathaniswa neminyaka emithathu yamabhethri ajwayelekile alahla.
Impilo yebhethri ye-acid acid:
I-Design Service Life of Holy-acid amabhethri ayiminyaka engu-3-5 nangaphezulu kweminyaka eyi-12, kepha le yimpilo yezemininingwane yezemibono. Kunokwehluka phakathi kwempilo yangempela yenkonzo kanye nethiyori. Udinga ukugcina ibhethri lakho le-lead-acid ngangokunokwenzeka ukuze uqinisekise ukuthi linokulahleka okuphansi kakhulu.
Izimo Zokusebenza:
Amabhethri e-lead-acid aluhlobo oluvame kakhulu lwebhethri olusetshenziswa ezimotweni nasezinye izinhlelo zokusebenza. Lawa mabhethri angathengwa kusuka cishe kunoma yimuphi umthengisi noma online, kuya ngosayizi nohlobo olufunayo.
Ukulungiswa kwebhethri okunemininingwane okunemininingwane kungabhekisa ku-athikili:
Uhlu Lokuhlola Ukulungiswa Kwebhethri lwe-Acid
Umehluko omkhulu phakathi kwalezi zinhlobo ezimbili zamabhethri inani lamandla agcinwe ngeyunithi ngayinye yesisindo. Ibhethri le-lead acid line-voltage ephakeme, okusho amandla amaningi emoto yakho ukuhambisa ngokushesha noma ukusebenzisa njengohlelo lokusekelayo lukagesi lwekhaya lakho / ibhizinisi. Ukuhola amabhethri e-acid nakho kuhlala isikhathi eside kunamabhethri e-alkaline, kepha ngoba awakhiqizi amandla amaningi ngeyunithi ngayinye yesisindo, abiza kakhulu!
Isikhathi sePosi: Jul-11-2022




