Ungakhokhisa kanjani ibhethri lejeli
Izicelo zebhethri
Ibhethri eliphephile ledatha:Akukho ukuvuza kwe-gel ku-gel bettery terminal, kuqinisekisa ukusebenzisa ephephile futhi kunokwethenjelwa.
Ibhethri lamahhala lesondlo:Ngenxa yawo wonke amagesi akhiqizwayo akhiqizwa emanzini, awudingi ukuphinda kwenziwe ngamanzi.
Uhlelo Lokukhipha Umoya:ingaphetha igesi eyeqile futhi yenze ingcindezi yomoya kuze kube luhlu olujwayelekile laphoibhethri lesithuthuthu se-gelUkwedlula zonke izingcindezi zangaphakathi ziphezulu kakhulu, kulokhu i-valve ephephile izovala ngokwayo, ngakho-ke ngeke kube khona okungeziwe kwegesi. Ukuchazwa Komkhiqizo.
Ayikho i-acid yamahhala:I-adchirb ekhethekile ye-adsorb electrorbte, ngakho-ke alikho i-acid yamahhala ye-acid i-acid yebhethri, khona-ke ibhethri le-VRLA lingafakwa endaweni ehlukahlukene.
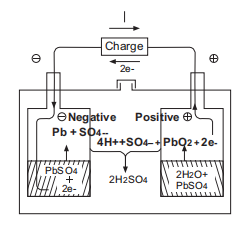
I-Chemical ReactionInvrla Battery iyalandela
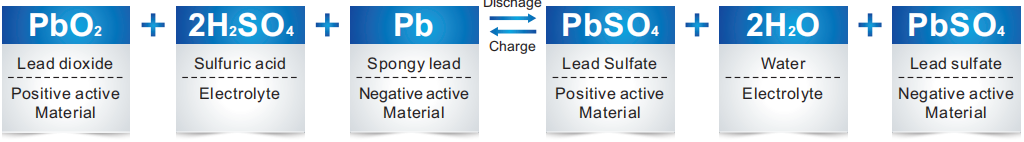
Ngenkathi ibhethri le-gel likhishwa, ukugcwala kwe-sulfuric acid kuncipha kancane kancane futhi kuholele i-sulfate ngaphansi kokuphendula phakathi kwe-ectrode enhle, i-spongy eholwa yi-electrode engemihle kanye ne-sulfuric acid ku-electorlyte.
Ngenkathi ishaja, uhola i-sulfate ku-electrode enhle nengelungile uguqulwa ukuze uhole i-dioxide kanye ne-spongy eholile, futhi ngokuhlukaniswa kwe-sulfuric acid, ukugcwala kwe-sulfuric acid kuzokhula.
Ngesikhathi sokushaja kokugcina kwebhethri lendabuko le-acid yendabuko, amanzi adliwa ukusabela kokuziphendukela kwemvelo kwe-hydrogen. Ngakho-ke kudinga isinxephezelo samanzi. Ngokusebenza kokuhola okunomswakama okunomswakama, kusabela ngokushesha ngo-oksijini, olawula ukwehla kwamanzi ngempumelelo.
Kuyafana namabhethri e-gel wendabuko kusukela ekuqaleni kwecala lokukhokha ngaphambi kwesigaba sokugcina, kepha uma kukhishwe ngokweqile futhi ngesikhathi sokugcina, amandla kagesi azoqala ukubola amanzi, i-electrode engemihle izoba sesimweni sokukhipha Ngoba umoya-mpilo ovela epuleti elihle uphendula ngokuhola okucacile kwepuleti elingesihle ne-sulfuric acid ye-electrolyte. Lokho kuvimbela ukuvela kwe-hydrogen kumapuleti amabi. Ingxenye ye-electrode engemihle esimweni sokukhipha iguqukela ekuholeni spongy ngenkathi ishaja.
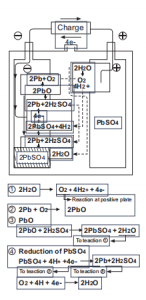
Inani le-spongy lead elakhiwe kusuka ekushaja ngokulingana kwenani le-sulfate lead njengomphumela wokuthola umoya-mpilo kusuka ku-electrode emihle, futhi kugcina sikwazi ukusayinaI-12v 12h ibhethri yeselula ye-gel. Ukusabela ngemuva kwesigaba sokugcina sokushaja kanye nokulingana kwamakhemikhali njengoba kungaphansi:
I-Fig.3: Ukusabela kusukela ekuqaleni kwecala lokuya ngaphambi kwesigaba sokugcina
Kungani Sikhethe US?
1. I-100% yokuhlola ukulethwa kokulethwa kokuqinisekisa ikhwalithi ezinzile nokusebenza okuthembekile.
2. Iplanethi yebhethri ye-PB-CA
I-3. Ukumelana okuphansi okuphansi, ukusebenza okuhle kakhulu kokukhipha.
I-4. Design ene-electrolyte egcwele amanzi, i-electrolyte eyanele, ukumelana okuphezulu kakhulu / kokukhipha ngokweqile.
5
6. Design Float Service Life: 3-5 iminyaka.
Isikhathi Sokuthumela: APR-07-2022

